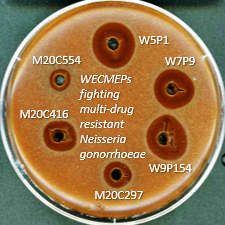
The Quality of Antimicrobial Susceptibility Test Discs and Implications for Clinical Outcomes
Disc diffusion is the primary method for assessing antibiotic activity in clinical microbiology laboratories, but its assay protocol is invalid and the quality of manufactured discs is unknown, potentially leading to adverse clinical outcomes. The lack of quality control standards and harmonization between EUCAST and CLSI suggests serious flaws in these systems.
AMR NEWS
Your Biweekly Source for Global AMR Insights!
Stay informed with the essential newsletter that brings together all the latest One Health news on antimicrobial resistance. Delivered straight to your inbox every two weeks, AMR NEWS provides a curated selection of international insights, key publications, and the latest updates in the fight against AMR.
Don’t miss out on staying ahead in the global AMR movement—subscribe now!